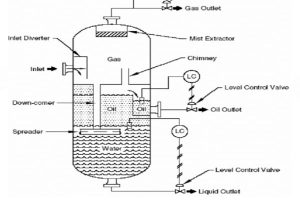
Five basic methods for dehydrating or "drying" natural gas
Glycol dehydrators, also known as gas dehydrators or TEG units, are used to remove water vapor from natural gas. The process of dehydration is important for two reasons.
First, water vapor can cause corrosion in pipelines and other gas-handling equipment. Second, water vapor can condense and freeze in low-temperature applications, which can result in clogged pipes and decreased efficiency.

Pictured above: Glycol dehydrator in service
All-natural gas wellstreams contain water vapor as they leave the reservoir. In many instances, free water is produced along with natural gas. Natural gas cools as it travels up the well bore to the surface as a result of pressure reduction and conduction of heat through the pipe to cooler formations.
Therefore, since the ability of gas to hold water vapor decreases as the gas temperature decreases, natural gas is nearly always saturated with respect to water vapor when it reaches surface equipment.
Additional cooling of the saturated gas will cause the formation of free water. Should the natural gas further cools into the hydrate range, hydrates will form, and serious equipment damage and stoppage of flow will occur. Thus, it is understandable why it is important to remove water vapor from natural gas.
The process for the removal of water vapor from natural gas is known as DEHYDRATION.
There are three principal reasons for dehydrating natural gas:
- Prevention of line plugging due to the formation of hydrates
- Prevention of reduction of line capacity due to the formation of free water (liquid)
- Elimination or retarding of corrosion in the pipeline
As stated before, two conditions must exist before hydrates can form – free water must be present in the gas stream, and the stream must be at or very near the hydrate temperature for the system pressure.
By reducing the water content of natural gas with dehydration, the operators can be sure that no free water with resulting hydrates will form in the pipeline until the gas in the line reaches its saturation temperature. A more detailed discussion on hydrates.
Free water in the pipeline occupies volume, reducing the line’s gas-carrying capacity. Any volume of water in the line means a loss in line capacity as the water will collect at low places in the line. Therefore, it is desirable to dehydrate the entering gas to a water vapor content that will prevent the formation of free water in the pipeline.
What are the different types of gas dehydration units available?
Glycol dehydrators and TEG units are the two most common types of gas dryers on the market today. Glycol dehydrators are well suited for low-volume applications, while TEG units are better for high-volume applications. Dehydrators are also available in a variety of sizes to accommodate different flow rates.
- Glycol dehydrators work by passing the gas through a solution of glycol and water. The glycol absorbs the water from the gas, and then the gas is passed through a series of filters to remove the glycol.
- Dehy units work similarly, but instead of glycol, they use a solid desiccant material to absorb the water from the gas. Dehydrators are also available with either forced or natural convection. Forced convection units have a blower that circulates the gas within the unit, while natural convection units rely on thermal currents to circulate the gas. When choosing a gas dehydration unit, it’s important to consider your specific needs and application.
Methods for Drying Natural Gas
There are five basic methods for dehydrating or “drying” natural gas:
- Cooling above hydrate expectancy temperature
- Compression followed by cooling
- Low-temperature separation
- Use of solid desiccants
- Use of liquid desiccants
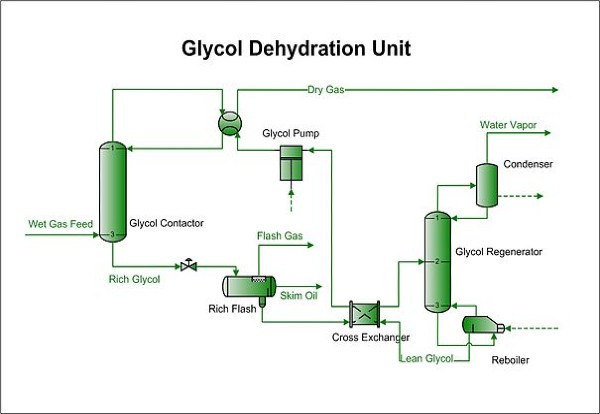
Pictured above: Glycol Dehydration Unit Diagram
Cooling
Cooling the stream is perhaps the simplest method of removing water vapor from natural gas; however, the process is limited by the hydrate-forming temperature for any given system pressure.
For example, a 0.6 specific gravity natural gas at 1000 psig has an average hydrate formation temperature of 64°F. This would limit the cooling to approximately 70°F because of the variation.
Considerable water vapor can be removed from the gas by cooling to 70°F. Assuming the gas ahead of the cooling system is at 100°F, the initial water content at 1000 psig is 61 lbs/mmscf.
The water content at 70°F is approximately 24 lbs/mmscf. By cooling from 100°F to 70°F the water content is reduced 37 lbs/mmscf or approximately 4.4 gallons/mmscf.
This method is often used when handling gas originating from extremely hot reservoirs such as those in volcanic regions or very deep formations. Air cooling and/or water cooling are commonly used as the preliminary steps to reduce the subsequent requirements for more complicated equipment for further removal of water vapor.
Compression and Cooling
Compression followed by cooling is a variation of the first process.
This process takes advantage of the effect that pressure has upon the saturation water content of natural gas.
The gas acts like a sponge in that the harder it is “squeezed”, the less water it can hold.
Therefore, a reduction of water content can be obtained by compressing the gas to a higher pressure and then cooling it.
The compression -process causes the gas to heat up, therefore cooling is required to bring it back or near the hydrate temperature.
The liquid water can then be removed with a separator. This method of water vapor removal is limited by the hydrate formation temperature just as the simple cooling method is. Any further reduction of the gas temperature will require additional dehydration or hydrate protection of some type.
Low-Temperature Separation
The Low-Temperature separation method can be used either where adequate pressure differential exists between the wellstream flowing pressure and the pipeline delivery pressure or where cooling by mechanical refrigeration can be used.
This former method makes use of the Joule-Thompson or auto-refrigeration effect that results from taking an appreciable pressure drop across a choke on the inlet to the low-temperature separator.
Normally this method requires an initial wellstream pressure of 1500 psig or higher with an available differential of at least 1000 psig.
When the wellhead pressure and available differential decline with reservoir age, the amount of dehydration and liquid recovery begins to reduce until it becomes necessary to use an alternate method or to supplement the cooling with a mechanical refrigeration unit.
There are two basic types of low-temperature separation units using the Joule-Thompson effect one with hydrate inhibitor injection and one without.
The unit with hydrate inhibitor injection takes full advantage of available auto-refrigeration. The injection of hydrate inhibitor into the wellstream ahead of the heat exchanger allows maximum cooling of the wellstream before pressure reduction.
This produces the lowest possible temperature in the low-temperature separator. The cold gas from the low-temperature separator is heat-exchanged with the inlet well stream.
If the inhibitor is an ethylene glycol or diethylene glycol solution, it can be separated and recovered in a re-concentrator for re-use.
If the inhibitor is an alcohol such as methanol, generally no attempt is made to recover it. The methanol is then dumped with the water phase from the low-temperature separator.
The unit without a hydrate inhibitor is very similar in design; however, the amount of wellstream cooling ahead of the choke must be controlled so the wellstream is still a few degrees above the hydrate temperature as it enters the choke body. Hydrates are produced and blown into the separator. The inlet wellstream is used to melt the hydrates by conducting it through a pipe coil in the separator near the region where the hydrates are collected. The wellstream is then cooled through heat exchange with the cold gas from the separator.
The low-temperature separation system using mechanical cooling is identical to the above system with hydrate inhibition except that refrigeration and a chiller are put in place of the expansion choke. The reduced separation temperature lowers the water content of the gas stream through condensation. The lower temperature also usually increases liquid hydrocarbon recovery, which will often amortize the equipment investments.
Any of the above low-temperature separation systems can be supplied by KW International. However, each application requires that the equipment be sized and designed specifically for its conditions. All details for a low-temperature separation system should be forwarded to the KW International Houston office for the best type of unit and the equipment required.
Solid Desiccants
There are several solid or “dry” desiccants used to remove water vapor from natural gas. The more common are activated alumina, silica gel, molecular sieves, and calcium chloride.
Except for calcium chloride, all these desiccants can be regenerated and re-used many times.
Also except for the calcium chloride units, solid desiccant gas dehydrators are multi-bed units that have the wet gas flowing through one or more beds to remove the water vapor while the other bed or beds are being regenerated and readied for placement on the wet gas stream when the bed or beds online reach near-saturation.
This alternate usage of towers or beds is the normal way solid desiccants are used. The requirement of multiple pressure vessels, switching valves, associated piping, and equipment make dry desiccant dehydrators the most expensive of all types of dehydrators.
However, they can reduce the water content of natural gas to an extremely low level. When the silica gel type desiccant is used, the unit can be designed to also extract marketable liquid hydrocarbons. These latter units are referred to as the “Short Cycle” hydrocarbon units.
As in the case of low-temperature separation units, each dry bed dehydrator is specifically designed for its own application. Based on your information and requirements, KW International can furnish your dry desiccant dehydrator needs.
The calcium chloride desiccant is deliquescent, i.e., it undergoes several successive chemical reactions with water vapor, gradually transforming from a solid to a brine solution. It is, therefore, used in a batch-type system.
The brine solution cannot be regenerated and is discarded. In most cases, only one specially designed tower is used and periodically taken off the line for recharging. Some installations use two towers so that one is always on the line while the other is being recharged. These units are infrequently used today because of the associated corrosion problems of calcium chloride brines and the fact that the water content of the outlet gas increases rapidly as the charge in the tower is spent.
Liquid Desiccants
The most widely used method of drying natural gas is the liquid desiccant unit.
Desiccants commonly used are methanol, ethylene glycol, diethylene glycol, triethylene glycol, and tetraethylene glycol.
Methanol, ethylene glycol, and diethylene glycol are normally used with injection systems as hydrate inhibitors. This is discussed under low-temperature separation.
Methanol and ethylene glycol are mostly used only in an emergency or temporary system because they are not easily recovered for reuse.
Diethylene glycol is commonly used in injection systems as it can be readily recovered, reconcentrated, and re-used. It is also used in the same manner as the other higher glycols, but it is not capable of producing as great a reduction in the water content of the gas as the higher molecular weight glycols.
There are three types of glycols used in dehydration systems. These are diethylene, triethylene, and tetraethylene glycol.
Ethylene glycol has been used in some special applications, but its vapor pressure is too high for use in conventional re-concentrators without experiencing very high losses.
Consequently, it is not considered as one of the major absorbents. Diethylene glycol is used because its vapor pressure is lower than ethylene glycol and it is not as soluble in liquid hydrocarbons as triethylene glycol or tetraethylene glycol.
Diethylene glycol is primarily used in glycol injection systems as a hydrate inhibitor, but it can also be used in conventional gas dehydrators where a limited dew point depression is acceptable.
Because of its lower decomposition temperature, diethylene glycol cannot be regenerated to as high percent reconcentration as triethylene glycol.
Diethylene glycol also has a price advantage over triethylene and tetraethylene glycol.
Triethylene glycol is the predominant glycol used in dehydration and has largely supplanted diethylene glycol for this purpose.
Because of its higher decomposition temperature and much lower vapor pressure, triethylene glycol can be more readily reconcentrated to a higher purity with a resultant increase in dew point depression without incurring decomposition and high losses from the still.
Recent improvements in reconcentrating equipment (making use of stripping gas) have resulted in achieving even higher purities with subsequent increases in dew point depressions.
Tetraethylene glycol has become commercially available for use as a liquid desiccant.
It has an even higher decomposition temperature than triethylene glycol. It can usually provide a slight increase in dew point depression over that obtained by triethylene glycol, using the same equipment.
The tetraethylene glycol must be reconcentrated at a higher reboiler temperature. It has another advantage over triethylene glycol in that there is a considerable reduction in glycol loss due to its lower vapor equilibrium at elevated contact temperatures due to the high temperature of the inlet gas. This is especially important where gas-glycol contact temperatures are above 120F. The major disadvantages are its high cost and its higher viscosity, which becomes a factor with low ambient air conditions and in cold climates. The recommended safe ranges of reconcentrated (reboiler) temperatures are as follows:
Diethylene Glycol 315F – 340F
Triethylene Glycol 340F – 400F
Tetraethylene Glycol 400F – 430F